Chem 1101 2014-J-3 Worksheet With Answers - The University Of Sydney - 2014 Page 20
ADVERTISEMENT
CHEM1101
2004-J-5
June 2004
Marks
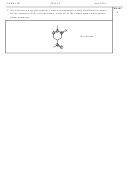
Complete the table below showing the number of valence electrons, a Lewis structure
5
and the predicted shape of each of the following species.
Formula
Number of
Lewis structure
Name of molecular shape
valence electrons
O
e.g. H
O
8
Bent (angular)
2
H
H
Cl
3 bonds and 1 lone pair
SOCl
26
on sulfur:
S
O
2
trigonal pyramdial
Cl
Cl
4 bonds and no lone
CCl
32
pairs on carbon:
Cl
C
Cl
4
tetrahedral
Cl
Which, if either, of SOCl
and CCl
will have a dipole moment?
SOCl
2
4
2
2
Using the following electronegativity data, decide which one or more of the oxides of
C, Te, Zn and Mg would be classified as containing ionic bonds. Briefly explain your
answer.
Element
Electronegativity
O
3.5
C
2.5
Te
2.1
Zn
1.4
Mg
1.2
An electronegativity (χ) difference > 2 is classified as ionic:
C: Δ χ = 3.5 – 2.5 = 1.0 not ionic
Te: Δ χ = 3.5 – 2.1 = 1.4 not ionic
Zn: Δ χ = 3.5 – 1.4 = 2.1 ionic
Mg: Δ χ = 3.5 – 1.2 = 2.3 ionic
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21








