Chem 1101 2014-J-3 Worksheet With Answers - The University Of Sydney - 2014 Page 19
ADVERTISEMENT
CHEM1101
2005-N-6
November 2005
Marks
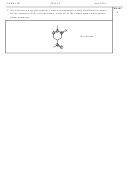
• Complete the table below showing the number of valence electrons, the Lewis
5
structure and the predicted shape of each of the following species.
Number of
Formula
Lewis structure
Geometry of species
valence electrons
H
N
H
e.g. NH
8
trigonal pyramidal
3
H
F
F
5 bonds and 1 lone pair
ClF
42
on chlorine:
5
F
Cl
F
square-based pyramid
F
2 bonds and 1 lone pair
–
NO
18
on nitrogen:
O
N
O
2
bent
–
Which of NH
, ClF
and NO
have a non-zero dipole moment?
3
5
2
All three have dipole moments
2
• What is the approximate value of the most intense wavelength emitted by the star
Proxima Centauri, which has a temperature of 2700 K?
The maximum intensity in a thermal spectrum is approximately at a transition
-23
-1
-1
energy ΔE= 4.5k
T where k
is Boltzmann’s constant (1.381 × 10
J K
mol
).
B
B
Using T = 2700 K, the transition energy can be calculated:
23
19
E
. 4
k 5
T
4
5 .
. 1 (
381
10
−
)
2700
. 1
68
10
−
J
Δ
=
==
×
×
×
=
×
B
To convert this energy into a wavelength, Planck’s relationship must be used:
34
8
hc
(
. 6
626
10
−
)(
. 2
998
10
)
×
×
6
. 1
18
10
m
or
1180
nm
−
λ
=
=
=
×
19
E
. 1
68
10
−
×
6 −
Answer:
. 1
18
10
m
or
1180
nm
×
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21








