Inorganic Molecular Shapes Workbook Page 2
ADVERTISEMENT
This topic concerns the 3 dimensional shape of molecules. Molecular shape is
very important when chemical reactions are considered. This is especially true
in Biochemical reactions. This aspect of molecular shape will be dealt with in
the medicinal topic of the Organic Chemistry unit. For now we will limit the
discussion to simple molecules with relatively few atoms.
You are already familiar with the basic shape of methane and other
hydrocarbons.
The methane molecule has a tetrahedral geometry and the angle between each
of the bonds is 109.5 degrees
The question is - why does
a methane molecule adopt
this particular shape?
The basic answer to this question is due to the electrons in the covalent bonds
holding the atoms together.
Each covalent bond is a shared pair of electrons. As electrons are negatively
charged, the electrons in a bond will repel the electrons in other bonds.
This diagram shows the covalent bonds in methane.
Each bonded pair of electrons will repel the other
three bonded pairs of electrons.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3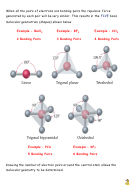 4
4 5
5 6
6 7
7 8
8 9
9 10
10








