Inorganic Molecular Shapes Workbook Page 9
ADVERTISEMENT
Look back at page three to see the bond angles formed when all the electron
pairs around the central atom are bonding pairs.
If a molecule, or ion, has
lone pairs of electrons
on the central atom, the
shapes are slightly distorted away from the regular shapes. This is because of
the extra repulsion caused by the lone pairs.
As a result of the extra repulsion, bond angles tend to be slightly less as the
bonds are “squeezed” together.
The three molecules above show decrease in bond angle as the number of
NON-BONDED electron pairs exert a greater repulsive effect on the bonded
pairs. The same effect is seen in other molecules.
Remember that lone pairs of electrons cause bond angles to vary from normal.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3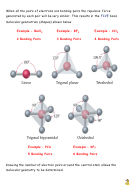 4
4 5
5 6
6 7
7 8
8 9
9 10
10








