Inorganic Molecular Shapes Workbook Page 4
ADVERTISEMENT
When all the pairs of electrons are bonding pairs the repulsive force
generated by each pair will be very similar. This results in the
FIVE
basic
molecular geometries (shapes) shown below
Example – CCl
Example - BeCl
Example – BF
4
2
3
4 Bonding Pairs
2 Bonding Pairs
3 Bonding Pairs
Example – SF
Example – PCl
6
5
6 Bonding Pairs
5 Bonding Pairs
Knowing the number of electron pairs around the central atom allows the
molecular geometry to be determined.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3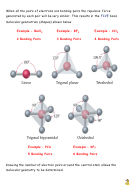 4
4 5
5 6
6 7
7 8
8 9
9 10
10








