Inorganic Molecular Shapes Workbook Page 8
ADVERTISEMENT
1. Go back to the question on page 5 and decide how many bonding and how
many non - bonding electron pairs each substance has.
In the exam you may be asked to draw a diagram of a molecule or ion which
clearly shows the shape of the molecule or the shape that the electron pairs
adopt. Your drawing must not look “FLAT” and must give an idea of the three
dimensional arrangement of the electron pairs or of the atoms.
The diagrams below show one way to do this.
Match the
electron pair
geometry of the molecules below to the shapes above.
a.
BrF
b.
PCl
c
AlBr
d.
XeF
e.
H
O
+
+
5
4
3
2
3
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3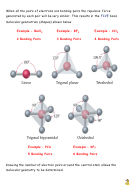 4
4 5
5 6
6 7
7 8
8 9
9 10
10








