Inorganic Molecular Shapes Workbook Page 6
ADVERTISEMENT
1. Determine the number of electron pairs in the following molecules or ions.
a.
H
O
b.
NH
c.
SF
d.
ICl
-
2
3
6
4
e.
BCl
f.
NF
g.
SiCl
h.
PF
3
3
4
5
i.
PH
j.
BeF
k.
IF
l.
SiCl
+
2-
4
2
5
6
Another important factor in determining molecular geometry is the nature of
the electron pairs.
Consider the bonding in the ammonia molecule,
NH
3
The electronic configuration of nitrogen is
1s
2s
2p
2
2
3
Nitrogen has three unpaired electrons in its valence shell . It is these electrons
which form the three bonds with the hydrogen atoms in ammonia. However,
nitrogen also has a pair of electrons in its valence shell – the 2 electrons in the
2s sub-shell. These are usually non – bonding electrons.
Ammonia has 4 pairs of electrons. Three are bonding pairs – hence three bonds,
and one lone pair {non bonding pair}.
The geometry of the
ELECTRONS
is
TETRAHEDRAL
but the geometry of the
ammonia molecule is a
TRIGONAL PYRAMID.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3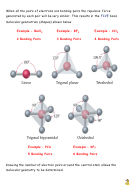 4
4 5
5 6
6 7
7 8
8 9
9 10
10








