Inorganic Molecular Shapes Workbook Page 3
ADVERTISEMENT
The theory known as valence shell electron pair repulsion
(VSEPR) is used to explain why molecules adopt a particular
shape.
The basic principle behind the theory states:
Consider the molecule shown below. It contains atom X which has three
covalent bonds to the atoms of Y.
In this diagram two of the bonds angles are 90 degrees
Y
and one is 180 degrees. This is an unfavourable geometry
as the repulsion of the bonded electrons is unequal –
there will be greater repulsion between the electron
Y
Y
pairs which are 90 degrees apart than between the
electron pairs which are 180 degrees apart.
Y
In this diagram all the bond angles are all 120 degrees
and so the electron pairs are as far apart from each
other as possible. This will be the lowest energy situation
for this molecule as the repulsive forces are pushed as
Y
Y
far apart as possible.
In this example atom X has three bonded pairs of electrons – atom X is
bonded to three other atoms. This is not always the case. Molecules can have
more or less bonded pairs than the molecule shown (e.g. methane has 4 bonded
pairs). The number of pairs of electrons a molecule has will dictate the shape
adopted by the molecule. In addition some molecules have non – bonded pairs
of electrons called
LONE PAIRS
which also influence molecular shape.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3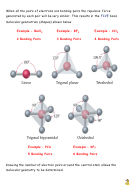 4
4 5
5 6
6 7
7 8
8 9
9 10
10








