Atoms, Molecules, And Ions Worksheet - Chapter 2 Page 12
ADVERTISEMENT
Chemical Formulas
• The subscript to the right of the
symbol of an element tells the
number of atoms of that element
in one molecule of the compound.
• Molecular compounds often
contain only nonmetals.
• The attraction between molecules
are often relatively weak,
explaining why gases and liquids
are common among molecular
substances.
• Carbon is typically listed first in
the formula, unless C is part of a
polyatomic ion.
Types of Formulas
• Empirical formulas
give the
lowest whole-number ratio of
atoms of each element in a
compound.
• Molecular formulas
give the
exact number of atoms of each
element in a compound.
• Structural formulas
show the
order in which atoms are
bonded.
• Perspective drawings
also show
the three-dimensional shape of
atoms in a compound.
12
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26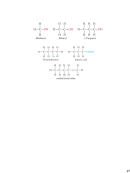 27
27








