Atoms, Molecules, And Ions Worksheet - Chapter 2 Page 7
ADVERTISEMENT
Atomic and Mass Numbers
Atomic number: equal to the number of protons in
the nucleus. All atoms of the same element have
the same number of protons. Denoted by “Z”.
Mass number: equal to the sum of the number of
protons and neutrons for an atom.
Atomic Mass
The mass of an atom in atomic mass units (amu) is the
total number of protons and neutrons in the atom.
A carbon (C) atom with 6 protons and 6 neutrons is
assigned a mass of exactly 12 amu.
Atomic mass unit (amu) is one-twelfth of the mass of
an atom of carbon with 6 protons and 6 neutrons.
-24
1 amu = 1.66054 × 10
g
23
1 g = 6.02214 × 10
amu
7
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26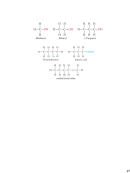 27
27








