Atoms, Molecules, And Ions Worksheet - Chapter 2 Page 24
ADVERTISEMENT
Nomenclature of Binary
Compounds (molecular)
Formed Between Nonmetals.
• The less electronegative atom
(farthest to left) is usually listed
first.
• A Greek prefix is used to denote the
number of atoms of each element in
the compound (mono- is not used
on the first element listed, however.)
• The ending on the more
electronegative element is changed
to -ide.
– CCl
: carbon tetrachloride
4
– CO
: carbon dioxide
2
– CO: carbon monoxide
– N
O
: dinitrogen tetroxide
2
4
• In nonmetal compounds containing
carbon, the C atom is listed first in
the chemical formula, followed by H.
24
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26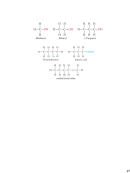 27
27








