Atoms, Molecules, And Ions Worksheet - Chapter 2 Page 9
ADVERTISEMENT
Atomic Masses of the Elements
•
Isotopic mass
is the mass in amu (u), of a particular
isotope of an element.
• Different isotopes of an element all react essentially
the same, so a weighted average of isotopic masses
can be used in calculations.
• The
atomic weight
is the weighted average mass, of
the naturally occurring element. It is calculated from
the isotopes of an element weighted by their relative
abundances.
. . .
Atomic weight = fraction
m
+ fraction
m
+
A
A
B
B
Σ
Atomic weight =
[(isotope mass)×(fractional isotope abundance)]
Average Atomic Mass = Atomic Weight
Periodicity
When one looks at the chemical properties of elements,
one notices a repeating pattern of reactivities.
9
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26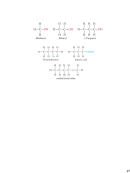 27
27








