Vsepr And Polarity Worksheet Page 6
ADVERTISEMENT
Chemistry 121 Problem set V Solutions - 6
O
−
(143 120)
O
O
−
=
− =
143
143 8 135pm
3
C
C
C
O
O
O
O
O
O
2 −
Order with respect to C−O bond length (longest to shortest): CH
OH > CO
> CO
> CO
3
3
2
7. ∆EN (C−O) = 3.4 − 2.5 = 0.9
C
O
The electronegativity vector is toward oxygen, i.e. oxygen has a higher electron density than carbon,
but the formal charges indicate that in CO, oxygen is deficient in electrons and carbon has an excess, which
would lead to a lower separation of charge and a smaller dipole moment than expected from electronegativity
values.
−
8. Cyanate, NCO
(i)
(ii)
(iii)
N
C
O
N
C
O
N
C
O
The most stable Lewis structure is (ii) with the negative charge on oxygen, though (i) with the negative charge
on nitrogen will contribute to the resonance hybrid, making cyanate a stable anion.
−
Fulminate, CNO
(i)
(ii)
(iii)
C
N
O
C
N
O
C
N
O
Carbon forms three bonds, under special conditions it will form three bonds and carry a single negative charge,
but such species are very reactive. Lewis structure (ii) is the only significant contributor to the resonance hybrid,
structures (i) and (iii) with two and three negative charges on carbon will contribute insignificantly to the
resonance hybrid.
Thus the cyanate ion with two Lewis structures contributing to the resonance hybrid (negative charge on oxygen
and nitrogen) would be expected to be reasonably stable, while the fulminate ion with only one significant
structure contributing to the resonance hybrid and with the negative charge on carbon would be expected to be
quite unstable.
−
9.
a) HSO
4
O
O
O
HO
S
O
H
O
S
O
HO
S
O
O
O
O
−
ClO
4
O
O
O
O
O
Cl
O
O
Cl
O
O
Cl
O
O
Cl
O
O
O
O
O
−
H
PO
2
4
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1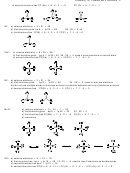 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10








