Vsepr And Polarity Worksheet Page 2
ADVERTISEMENT
Chemistry 121 Problem set V Solutions - 2
c) second structure has FC (Xe) = 8 - 2 - 3 = +3
FC (O) = 6 - 1 - 6 = -1
O
O
O
3
Xe
Xe
Xe
O
O
O
O
O
O
NF
a) valence electrons = 5 + 21 = 26
3
b) first structure gives bp 6 + lp 20 = 26
26 - 26 = 0 ok
c) first structure has FC(N) = 5 - 3 - 2 = 0 FC(F) = 7 - 1 - 6 = 0
F
N
F
F
XeF
a) valence electrons = 8 + 28 = 36
4
b) first structure gives
bp 8 + lp 24 = 32 36 - 32 = 4 need 4 more electrons on central atom
c) second structure has FC(Xe) = 8 - 4 - 4 = 0 FC(F) = 7 - 1 - 6 = 0
F
F
F
Xe
F
F
Xe
F
F
F
BF
a) valence electrons = 3 + 21 = 24
3
b) first structure gives
bp 6 + lp 20 = 26
24 - 26 = -2 need to lose two electrons
but do not use multiple bonds in this case as boron is electron deficient and only 6 electrons on boron
c) second structure has
FC(B) = 3 - 3 - 0 = 0 FC(F) = 7 - 1 - 6 = 0
F
F
B
B
F
F
F
F
-
MnO
a) valence electrons = 7 + 24 + 1 = 32
4
b) first structure gives
bp 8 + lp 24 = 32
32 - 32 = 0
c) first structure has FC(Mn) = 7 - 4 - 0 = +3
FC(O) = 6 - 1 - 6 = -1
d) resonance
O
O
O
3
O
Mn
O
O
Mn
O
O
Mn
O
O
O
O
O
O
O
O
O
Mn
O
O
Mn
O
O
Mn O
O
Mn
O
O
O
O
O
SO
a) valence electrons = 6 + 12 = 18
2
b) first structure has bp 4 + lp 16 = 20 18 - 20 = -2 need to lose 2 electrons as double bonds
c) second structure has bp 6 + lp 12 = 18
d) second structure has FC(S) = 6 - 3 - 2 = +1 FC(-O) = 6 - 1 - 6 = -1 FC(=O) = 6 - 2 - 4 = 0
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1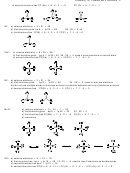 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10








