Vsepr And Polarity Worksheet Page 7
ADVERTISEMENT
Chemistry 121 Problem set V Solutions - 7
O
O
HO
P
O
HO
P
O
OH
OH
−
thus ClO
has the most Lewis structures contributing to the resonance hybrid and the negative charge is
4
−
delocalized to the greatest extent, making it the weakest base (and the most stable anion). H
PO
has
2
4
the least contributors to the resonance hybrid and the negative charge is delocalized to the smallest
extent, making it the least stable and thus the most basic.
−
−
−
So the order of increasing basicity is: ClO
< HSO
< H
PO
4
4
2
4
−
b) ClO
2
O
Cl
O
O
Cl
O
−
ClO
3
O
O
O
Cl
Cl
Cl
O
O
O
O
O
O
−
ClO
Cl
O
−
thus ClO
is the least stable with the negative charge localized on one oxygen, making it the most basic;
−
ClO
is the most stable with the negative charge delocalized on three oxygens, making it the least basic.
3
−
−
−
So the order of increasing basicity is: ClO
< ClO
< ClO
3
2
∆EN(F-S) = 1.40 and ∆EN(lp-S) = 1.08
10.
+
−
SF
SF
SF
SF
2
3
3
4
F
F
S
F
F
S
F
F
S
F
F
S
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
S
S
S
S
net vector = 0.32
net vector = 0.32
net vector = 0.32
net vector = 0.32
permanent dipole
permanent dipole
permanent dipole
permanent dipole
+
−
SF
SF
SF
5
5
6
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1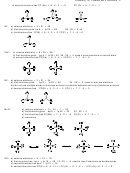 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10








