Vsepr And Polarity Worksheet Page 9
ADVERTISEMENT
Chemistry 121 Problem set V Solutions - 9
12.
Since Sb and F have different electronegativities, a nonpolar species will have all the electronegativity
vectors cancel. ∆EN(F-Sb) = 1.93 and ∆EN(lp-Sb) = 1.61
So, working through the possible species with an even number of valence electrons and zero or a single
charge we have:
SbF, the single vector cannot cancel so it is polar.
+
−
+
SbF
SbF
SbF
SbF
2
2
3
4
F
F
Sb
F
F
Sb
F
F
Sb
F
F
Sb
F
F
F
F
F
F
F
F
F
F
F
F
F
F
Sb
Sb
Sb
net vector = 0.28
net vector = 0.28
net vector = 0.28
vectors cancel
permanent dipole
permanent dipole
permanent dipole
no permanent dipole
−
+
−
SbF
SbF
SbF
SbF
4
5
6
6
F
F
F
F
F
Sb
F
Sb
F
F
I
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
Sb
net vector = 0.28
vectors cancel
can not exist
vectors cancel
permanent dipole
no permanent dipole
no permanent dipole
13. The only VSEPR shape where all adjacent F-X-F angles are 90º is the octahedron, so we want XF
. The
6
Lewis structure must have no lone pairs on the central atom.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1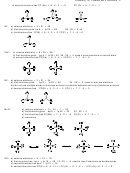 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10








