Vsepr And Polarity Worksheet Page 5
ADVERTISEMENT
Chemistry 121 Problem set V Solutions - 5
f) nonpolar
F
F
C
F
C
F
F
F
F
F
4.
(a)
(b)
(c)
N
N
O
N
N
O
N
N
O
(c) is the least stable as it has the most formal charge (including two negatives on one nitrogen)
(b) is the most stable as the negative charge is on oxygen
(a) is less stable than (b) with the negative charge on nitrogen
Order of contribution to resonance hybrid is (b) > (a) >> (c)
The resonance hybrid will have the largest contribution from (b) and a smaller contribution from (a) so the
N-N bond will have an electron density between a triple and a double bond and thus its length will be
between 110 and 120 ppm, in fact it is 112 ppm. The N-O bond will have an electron density between a
single and a double bond and so its length will be between 115 and 147 ppm, in fact it is 119 ppm.
5a)
(i)
(ii)
(iii)
H
H
H
C
N
N
C
N
N
C
N
N
H
H
H
Lewis structure (iii) is not legitimate as carbon is forming 5 bonds and has 10 valence electrons. Carbon only
has orbitals for 8 valence electrons.
(i) is the most stable as the negative charge is on nitrogen
(ii) is less stable than (i) with the negative charge on carbon
Order of contribution to resonance hybrid is (i) > (ii)
b) Hybridization
H
H
C
N
N
C
N
N
H
H
2
2
3
sp
sp
sp
sp
sp
sp
c) (i) H−C−N 120º, C−N−N 180º; (ii) H−C−N 109.5º, C−N−N 180º
6.
Species
C−O bond length
113 pm
C
O
120 pm
O
C
O
H
143 pm
H
C
O
H
H
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1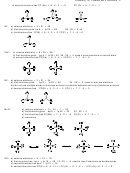 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10








