Vsepr And Polarity Worksheet Page 10
ADVERTISEMENT
Chemistry 121 Problem set V Solutions - 10
F
F
F
F
F
F
X
F
F
F
F
F
F
which gives 6(6) + 6(2) = 48 valence electrons
thus: (valence electrons on X) + 6(7) = 48 and so: (valence electrons on X) = 6
X can be S, Se, Te but not O.
The only VSEPR shape where all adjacent F-X-F angles are slightly less than 90º is the octahedron with
one lone pair on the central atom, so we want XF
.
5
F
F
F
F
F
F
F
F
F
F
which gives 5(6) + 2 + 5(2) = 42 valence electrons
thus: (valence electrons on X) + 5(7) = 42 and so: (valence electrons on X) = 7
X can be Cl, Br, I but not F.
14.
The VSEPR shape where XF
has a permanent dipole can not be a tetrahedron (4 bp) or an octahedron
4
with two lone pairs (4 bp and 2 lp) as in each case all the electronegativity vectors will cancel. It can not
be a trigonal planar as this shape has only 3 regions of electron density and we have 4 bp. The VSEPR
shape can only be a trigonal bipyramid.
F
F
X
F
X
F
F
F
F
F
which gives 4(6) + 2 + 4(2) = 34 valence electrons
thus: (valence electrons on X) + 4(7) = 34 and so: (valence electrons on X) = 6
X can be S, Se, Te but not O (as oxygen can have a maximum of 8 electrons on the central atom).
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1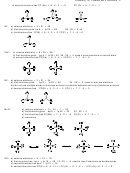 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10








