Vsepr And Polarity Worksheet Page 8
ADVERTISEMENT
Chemistry 121 Problem set V Solutions - 8
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
S
vectors cancel
net vector = 0.32
vectors cancel
no permanent dipole
permanent dipole
no permanent dipole
11 a) To obtain a square planar arrangement, we need an octahedral VSEPR shape (6 electron pairs) with two
lone pairs on the central atom. Thus
F
F
I
F
F
−
FC (I) = 7 − 4 − 4 = −1 thus the formula is IF
4
b) To obtain a pyramidal arrangement, we need an tetrahedral VSEPR shape (4 electron pairs) with one
lone pair on the central atom. Thus
F
I
F
F
2 +
FC (I) = 7 − 2 − 3 = +2 thus the formula is IF
3
c) To obtain a T-shaped arrangement, we need an trigonal bipyramid VSEPR shape (5 electron pairs)
with two lone pairs on the central atom. Thus
F
I
F
F
FC (I) = 7 − 4 − 3 = 0 thus the formula is IF
3
d) To obtain a linear arrangement, we need an trigonal bipyramid VSEPR shape (5 electron pairs) with
three lone pairs on the central atom. Thus
F
I
F
−
FC (I) = 7 − 6 − 2 = −1 thus the formula is IF
2
e) To obtain a square pyramid arrangement, we need an octahedral VSEPR shape (6 electron pairs) with
one lone pair on the central atom. Thus
F
F
I
F
F
F
FC (I) = 7 − 2 − 5 = 0 thus the formula is IF
5
f) To obtain a octahedral arrangement, we need an octahedral VSEPR shape (6 electron pairs). Thus
F
F
F
I
F
F
F
+
FC (I) = 7 − 0 − 6 = +1 thus the formula is IF
6
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1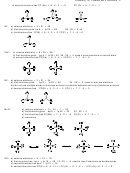 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10








