Vsepr And Polarity Worksheet Page 4
ADVERTISEMENT
Chemistry 121 Problem set V Solutions - 4
e) third structure: ∆EN (F-O) = 0.54 and resultant ∆EN (F-O) vector between F-O bonds; ∆EN (lp-O) =
0.22 and resultant ∆EN (lp-O) vector between lp-O bonds; both resultant vectors come close to cancelling
(0.54 − 0.26 = 0.28).
f) weakly polar, ∆EN = 0.3 (the dipole moment is 0.3 D)
F
O
O
O
F
F
F
CH
Cl
a) valence electrons = 4 + 14 + 2 = 20
2
2
b) first structure has bp 8 + lp 12 = 20
c) VSEPR (central atom) bp 4 + lp 0 = 4
VSEPR shape is tetrahedral (second structure)
d) Molecular shape is tetrahedral
e) third structure: ∆EN (Cl-C) = 0.61 and resultant ∆EN (Cl-C) vector between Cl-C bonds;
∆EN (C-H) = 0.35 and resultant ∆EN (C-H) vector between C-H bonds; resultant vectors are
additive (0.61+0.35 = 0.96).
f) polar, ∆EN = 0.96 (the dipole moment is 1.6 D)
H
H
Cl
Cl
C
H
C
Cl
Cl
H
NCl
a) valence electrons = 5 + 21 = 26
3
b) first structure has bp 6 + lp 20 = 26
c) VSEPR (central atom) bp 3 + lp 1 = 4
VSEPR shape is tetrahedral (second structure)
d) Molecular shape is trigonal pyramidal
e) third structure: ∆EN (N-Cl) = 0.12 and resultant small ∆EN (N-Cl) vector between N-Cl bonds;
∆EN (lp-N) =0.66 and ∆EN (lp-N) vector overcomes the resultant from the ∆EN (N-Cl) vectors
(0.66 – 0.12 = 0.54).
f) polar, ∆EN = 0.54 (the dipole moment is 0.39 D)
Cl
Cl
N
N
Cl
Cl
N
Cl
Cl
CHF
a) valence electrons = 4 + 21 + 1 = 26
3
b) first structure has bp 8 + lp 18 = 26
c) VSEPR (central atom) bp 4 + lp 0 = 4
VSEPR shape is tetrahedral (second structure)
d) Molecular shape is tetrahedral
e) third structure: ∆EN (F-C) = 1.43 and resultant ∆EN (F-C) vector between F-C bonds; ∆EN (C-H) =
0.34 and ∆EN (C-H) vector adds to the resultant from the ∆EN (F-C) vectors (0.35 + 1.43 = 1.78).
f) strongly polar, ∆EN = 1.78 (the dipole moment is 1.65 D)
H
H
C
F
C
F
F
F
F
F
CF
a) valence electrons = 4 + 28 = 32
4
b) first structure has bp 8 + lp 24 = 32
c) VSEPR (central atom) bp 4 + lp 0 = 4
VSEPR shape is tetrahedral (second structure)
d) Molecular shape is tetrahedral
e) third structure: ∆EN (F-C) = 1.43 and all ∆EN (F-C) vectors cancel.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1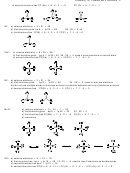 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10








