Vsepr And Polarity Worksheet Page 3
ADVERTISEMENT
Chemistry 121 Problem set V Solutions - 3
e) resonance (the 3d obitals on S are close in energy to the 3p orbitals and S can take more than 8
electrons)
S
S
O
S
O
O
O
O
O
S
S
S
O
O
O
O
O
O
O
a) valence electrons = 18
3
b) first structure has bp 4 + lp 16 = 20 18 - 20 = -2 need to lose 2 electrons as double bonds
c) second structure has bp 6 + lp 12 = 18
d) second structure has FC(-O=) = 6 - 3 - 2 = +1 FC(-O) = 6 - 1 - 6 = -1 FC(=O) = 6 -2 - 4 = 0
e) resonance (no two double bond hybrid as the lowest energy empty orbitals are too high in energy and
so oxygen can only take 8 electrons, no 2d orbitals.
O
O
O
O
O
O
O
O
O
O
O
O
3.
XeF
a) valence electrons = 8 + 28 = 36
4
b) first structure has bp 8 + lp 28 = 36 (problem 12)
c) VSEPR (central atom) bp 4 + lp 2 = 6
VSEPR shape is octahedral (second structure)
d) Molecular shape is square planar
e) third structure: ∆EN (F-Xe) = 1.38 and all 4 ∆EN (F-Xe) vectors cancel; ∆EN (lp-Xe) = 1.1 and the
two lp ∆EN vectors cancel
f) nonpolar
F
F
F
Xe
F
Xe
F
F
F
F
SF
a) valence electrons = 6 + 42 = 48
6
b) first structure has bp 12 + lp 36 = 48
c) VSEPR (central atom) bp 6 + lp 0 = 6
VSEPR shape is octahedral (second structure)
d) Molecular shape is octahedral
e) third structure: ∆EN (F-S) = 1.4 and all 6 ∆EN (F-S) vectors cancel
f) nonpolar
F
F
F
F
F
F
S
S
F
F
F
F
F
F
OF
a) valence electrons = 6 + 14 = 20
2
b) first structure has bp 4 + lp 16 = 20
c) VSEPR (central atom) bp 2 + lp 2 = 4
VSEPR shape is tetrahedral (second structure)
d) Molecular shape is bent
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1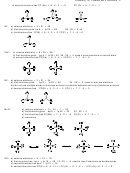 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10








