Quality Assurance Project Plan Including Sampling And Analysis Plan Page 34
ADVERTISEMENT
Hunters Point Shipyard Parcel F ESTCP Demonstration Plan
Appendix A: Quality Assurance Project Plan
cores that come from one given plot. Homogenize the resulting 500-g sample and remove two
100-g subsamples for the desorption tests. These two 100-g subsamples should be placed into
separate pre-cleaned and labeled 4 oz. glass jars.]
•
The remainder of the core will be archived;
•
Document sediment subsamples by recording the following information: Date sediments were
subsampled, ID code or number for each subsample, and sample allocation information (analyses
performed).
At the conclusion of the project, sediment waste will be classified as potential hazardous waste and
disposed of through procedures outlined by Stanford’s Environmental Health and Safety program.
A.3.3.3.7
Sample Homogenization
Sample homogenization is a critical activity and must be conducted to ensure that the homogenate and
aliquots are representative of the field material. For sediment samples, the sample homogenization
referenced in Section A.3.3.3.6 will be accomplished by placing the wet sediment in a clean, glass
container and mixed thoroughly by hand until a homogeneous color and texture is achieved.
Homogenization of tissue samples is described in Sections A.3.4.2.1 and A.3.4.4.3.
A.3.3.4
Field Sample Preservation, Packaging, and Shipment
During the sampling day, samples collected for the ESTCP DP will be preserved by placing the
containers in coolers immediately after collection. At the end of the sampling day, all field samples that
are to be shipped overnight will be packaged in coolers and shipped with the appropriate chain-of-custody
(COC) forms as described in Section A.3.3.5. Each of these coolers will also contain a temperature cooler
blank so that the receiving laboratory may verify sample temperature upon receipt. Sediment cores will be
stored upright.
A.3.3.5
Chain-of-Custody Records
Sample custody records are the administrative records associated with the physical possession and/or
storage history of each individual sample from the purchase and preparation of each sample container and
sampling apparatus to the final analytical result and sample disposal.
Sample custody will be documented throughout collection, shipping, analysis, and disposal of the sample.
Samples will not be left unattended unless properly secured. The sample custody form provides a record
of the samples collected and analyses requested. If more than one cooler is sent in one shipment to the
laboratory, then each cooler will contain a separate custody record for the samples in that cooler. In
addition, the outside of the coolers will be marked to indicate the number of coolers in the shipment (e.g.,
1 of 2, 2 of 2). All coolers must be shipped under a bill of lading that identifies the total number of
coolers in the shipment. Separate tracking numbers will be assigned to each cooler. Specifically for
sample archives sent to BDO, sample custody procedures will be in accordance with the BDO SOPs 6-
010, Sample Receipt, Custody, and Handling.
Each analytical laboratory must have a formal, documented system designed to provide sufficient infor-
mation to reconstruct the history of each sample, including preparation of sampling containers, sample
collection and shipment, receipt, distribution, analysis, storage or disposal, and data reporting within the
laboratory. Laboratory documentation must provide a record of custody for each sample (versus a sample
batch) throughout processing, analysis, and disposal.
Page A-
34
of A-67
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Business
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26 27
27 28
28 29
29 30
30 31
31 32
32 33
33 34
34 35
35 36
36 37
37 38
38 39
39 40
40 41
41 42
42 43
43 44
44 45
45 46
46 47
47 48
48 49
49 50
50 51
51 52
52 53
53 54
54 55
55 56
56 57
57 58
58 59
59 60
60 61
61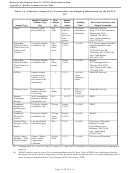 62
62 63
63 64
64 65
65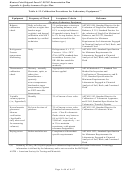 66
66 67
67








