Quality Assurance Project Plan Including Sampling And Analysis Plan Page 35
ADVERTISEMENT
Hunters Point Shipyard Parcel F ESTCP Demonstration Plan
Appendix A: Quality Assurance Project Plan
The custody form summarizes the samples collected and analyses requested. The custody form tracks
sample release from the field to the initial receiving laboratory. Each sample custody form will be signed
by the person relinquishing samples once that person has verified that the custody form is accurate; i.e.,
that all samples present in the shipping container are listed on the form, and that the sample descriptions,
requested analytical methods, and sampling dates are accurate. The original sample custody forms
accompany the samples; the shipper will keep a copy. Upon receipt at the sample destination, sample
custody forms will be signed by the person receiving the samples once that person has verified that all
samples identified on the custody forms are present in the shipping container. Any discrepancies will be
noted on the form (in addition to any internal laboratory documentation policy) and the sample receiver
will immediately contact the Project Manager to report missing, broken, or compromised samples.
Samples are considered to be in a person's custody if:
•
The samples are in a person's actual possession;
•
The samples are in a person's view after being in that person's possession;
•
The samples were in a person's possession and then were locked or sealed up to prevent
tampering; or,
•
The samples are in a secure area.
A.3.3.6
Sample Receipt
Immediately upon receipt by a laboratory, the condition of samples must be assessed and documented.
The contents of the shipping container must be checked against the information on the custody form for
anomalies. If any discrepancies are noted, or if laboratory acceptance criteria or project-specific criteria
are not met, the laboratory must contact the Project Manager for resolution of the problem. The dis-
crepancy, its resolution, and the identity of the person contacted must be documented in the project file.
The following conditions may cause sample data to be unusable and must be communicated to the
laboratory team leader:
•
The integrity of the samples is compromised (e.g., leaks, cracks, grossly contaminated
container exteriors or shipping cooler interiors, obvious odors, etc.);
•
The identity of the container cannot be verified;
•
The proper preservation of the container cannot be established;
•
Incomplete sample custody forms (e.g., the sample collector is not documented or the custody
forms are not signed and dated by the person who relinquished the samples);
•
The sample collector did not relinquish the samples; and,
•
Required sample temperatures were not maintained during transport.
The custodian must verify that sample conditions, amounts, and containers meet the requirements for the
sample and matrix (Table A-6). A unique sample identifier must be assigned to each sample container
received at the laboratory, including multiple containers of the same sample.
Page A-
35
of A-67
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Business
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26 27
27 28
28 29
29 30
30 31
31 32
32 33
33 34
34 35
35 36
36 37
37 38
38 39
39 40
40 41
41 42
42 43
43 44
44 45
45 46
46 47
47 48
48 49
49 50
50 51
51 52
52 53
53 54
54 55
55 56
56 57
57 58
58 59
59 60
60 61
61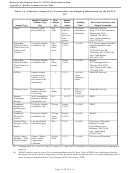 62
62 63
63 64
64 65
65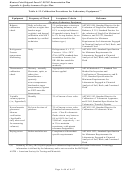 66
66 67
67








