Instructional Manual For Clarification Of Startup In Source Categories Affected By New Source Performance Standards - U.s. Environmental Protection Agency - 1979 Page 37
ADVERTISEMENT
 1
1  2
2  3
3  4
4  5
5  6
6  7
7  8
8  9
9  10
10  11
11  12
12  13
13  14
14  15
15  16
16  17
17  18
18  19
19  20
20  21
21  22
22  23
23  24
24  25
25  26
26  27
27  28
28  29
29  30
30  31
31  32
32  33
33  34
34  35
35  36
36  37
37  38
38  39
39  40
40  41
41  42
42  43
43  44
44  45
45  46
46  47
47  48
48  49
49  50
50  51
51  52
52  53
53  54
54  55
55  56
56  57
57  58
58  59
59  60
60  61
61  62
62  63
63  64
64  65
65 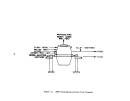 66
66  67
67  68
68  69
69  70
70  71
71  72
72  73
73  74
74  75
75  76
76  77
77  78
78  79
79  80
80  81
81  82
82  83
83  84
84  85
85  86
86  87
87  88
88  89
89  90
90  91
91  92
92  93
93  94
94  95
95  96
96  97
97  98
98  99
99  100
100  101
101  102
102  103
103  104
104  105
105  106
106  107
107  108
108  109
109  110
110  111
111  112
112  113
113  114
114  115
115  116
116  117
117  118
118  119
119  120
120  121
121  122
122  123
123  124
124  125
125  126
126  127
127  128
128  129
129  130
130  131
131  132
132  133
133  134
134  135
135  136
136  137
137  138
138  139
139  140
140  141
141  142
142  143
143  144
144  145
145  146
146 Introduction
SULFURIC ACID PLANTS .:. SUBPART H
§60.80 ... 60.85
Facilities affected by this regulation are those plants producing sulfuric
acid by the contact process by burning elemental sulfur, alkylation acid, hydro-
gen sulfide, organic sulfides and mercaptans, or acid sludge.
Facilities which
convert sulfur dioxide or other sulfur compounds to sulfuric acid primarily' as
a means of preventing emissions of the former are not subject to the regulation.
Emissions of gaseous sulfur dioxide cannot exceed
2
kg per metric ton (4.0
lb/ton) of 100 percent sulfuric acid.
Acid mist emissions, expressed as sul-
, furic acid, cannot exceed 0.075 kg per metric ton (0.15 lb/ton) of 100 percent
acid produced.
Opacity must be less than 10 percent.
In addition, continuous
monitoring for sulfur dioxide is required.
Sources constructed, reconstructed
or modified after August 17, 1971, are subject to the regulations.
Process Description
Three methods used to manufacture sulfuric acid
(H2S0~)
are the contact,
spent acid, and metallurgical processes, the first two of which are shown
in Figure 6.
Spent acid utilization occurs at petroleum refineries where
the acid is available.
The metallurgical process is employed as a sulfur
dioxide abatement method at plants roasting metallic sulfide ores and for
this reason is not subject to the regulation.
The most widely used manufacturing method is the contact process, in which
sulfur is burned to produce sulfur dioxide.
Sulfur is transported to the plant
in either the molten or elemental state; if not shipped in the molten state, the
sulfur is melted and filtered prior to burning.
Combustion air required for
burning is dried with 93 to 98 percent acid in drying towers and then fed to
the sulfur burner.
Before the sulfur dioxide (S02) gas can be introduced to the
converter, it must be cooled to about 427
0
C (800
0
F), the minimum temperature
at which the catalyst (usually diatomaceous earth impregnated with vanadium
pentoxide) will hasten the chemical reaction.
Heat is usually recovered in a
waste heat boiler, where steam is produced, and a heat exchanger, in which sul-
fur trioxide (S03) from the converter passes through and S02 surrounds the ex-
changer tubes.
The·S02 gases are purified by filtration prior to introduction
to the converter.
S02 is oxidized to S03 in the converter usually in four
stages with most (-75 percent) of the conversion taking place in the first
stage.
The temperatures are critical at the point of introduction to the next
pass and hot gases are again used to provide heat to other points in the plant.
The S03 can then be absorbed in an oleum tower (for production of oleums or S03
in
H2S0~)
and/or an absorbing tower (for production of
H2S0~
in water).
The
acids are then cooled and pumped to storage and the absorbing towers.
Best
25
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Legal