Review Of Organic Chemistry Page 11
ADVERTISEMENT
Organic Chemistry I Review: Highlights of Key Reactions, Mechanisms, and Principles
11
Chapter 7 Reactions and Mechanisms, Review
E2
Br
Br
Mech:
On
CH
3
NaOCH
3
R-X,
+
H OCH
H
(Normal
3
Normal
OCH
3
base)
H
+
H OCH
+ Br
Base
3
Notes
1. Trans hydrogen required for E2
2. Zaytsev elimination with normal bases
3. For 3º R-X, E2 only. But with 2º R-X, S
2 competes (and usually prevails)
N
4. Lots of “normal base” anions.
E2,
Br
Mech:
Br
H
NEt
or
2
NEt
3
3
On
C
+ Et
NH Br
KOC(CH
)
3
3
3
H
R-X, Bulky
(Bulky
Base
bases)
Notes:
1. Hoffman elimination with Bulky Bases
2. E2 dominates over S
2 for not only 3º R-X but also 2º R-X
N
3. Memorize NEt
and KOC(CH
)
as bulky bases.
3
3
3
Acid-
OH
Catalyzed
H
SO
2
4
+
H OH
E1-
Elimination
Of
Mech
Alcohols
OH
2
H
SO
OH
-H
O
2
4
2
Deprotonation
H
Protonation
Elimination
H
+ HSO
H
HSO
4
4
+ H
SO
+ OH
2
4
2
Notes:
1. Zaytsev elimination
2. Cationic intermediate means 3º > 2º > 1º
3. 3-Step mechanism
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10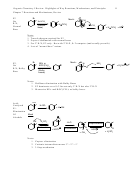 11
11 12
12 13
13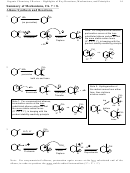 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26 27
27 28
28 29
29 30
30 31
31 32
32 33
33 34
34








