Review Of Organic Chemistry Page 24
ADVERTISEMENT
Organic Chemistry I Review: Highlights of Key Reactions, Mechanisms, and Principles
24
Ch. 16 Aromatic Compounds
16.1,2 Structure and Unique Properties of Benzene
C
H
6
6
C
B
A
2 Resonance Structures
Notes on Pictures and Structural Features
2
1. All 6 carbons are sp
, with one p orbital each
2. 120º angles, so all 6 carbons and each of their attached hydrogens are all co-planar.
3. Perfectly flat.
4. Perfect 120º angles, no angle strain whatsoever
5. Complete symmetry
6. Each C-C bond is equal in length and strength
7. Each C-C bond is longer than a normal double but shorter than a normal single bond
Normal Bond Lengths:
C-C: 1.54A
C=C: 1.34 A
Benzene CC: 1.39A
• “1.5” bonds, as we see from resonance.
1. Molecular Orbital for Benzene
Antibonding
Mix to Make
6 Molecular
Orbitals
Benzene: 6 p's
Nonbonding
Bonding
• All and only the bonding molecular orbitals are completely filled. Special stability
• But how can you know what the molecular orbitals will look like for other rings?
2
Frost Diagram/Polygon Rule: For a complete ring of sp
centers,
1. Draw the ring/polygon with a vertex down, basically inside what would be a circle
2. Each apex represents a molecular orbital
3. A horizontal line through the middle of the ring provides the non-bonding reference point
4. Populate the MO’s as needed depending on how many π-electrons are available
Molecular Orbital Rules for a cyclic π-system:
1. If all and only bonding molecular orbitals are occupied good (“aromatic”)
2. If any nonbonding or antibonding MO’s are occupied, or if any bonding MO’s are not
completely occupied bad, poor stability (“antiaromatic”)
• Below nonbonding line bonding
• Above nonbonding line antibonding
• On nonbonding line nonbonding
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10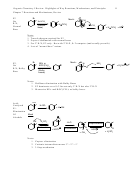 11
11 12
12 13
13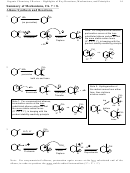 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26 27
27 28
28 29
29 30
30 31
31 32
32 33
33 34
34








