Review Of Organic Chemistry Page 18
ADVERTISEMENT
Organic Chemistry I Review: Highlights of Key Reactions, Mechanisms, and Principles
18
9. Bond Rotation Barrier: Bonds that look like singles but are actually between conjugated have
much larger rotation barriers than ordinary single bonds
• Because in the process of rotating, the p-overlap and its associated stability would be
temporarily lost
2
2
10. Hybridization: Conjugated sp
atoms have both sp
and p orbitals. You should always be able
to classify the hybridization of lone pairs on nitrogen and oxygen.
3
3
• Isolated oxygens or nitrogens: sp
atom hybridization, sp
lone-pair hybridization, and
tetrahedral, 109º bond angles
2
• Conjugated nitrogens: sp
atom hybridization, p lone-pair hybridization (needed for
conjugation), and 120º bond angles
2
• Conjugated oxygens: sp
atom hybridization, one p lone-pair hybridization (needed for
2
conjugation), one sp
lone-pair, and 120º bond angles
15.2 Diene Stability and the Stability of other Acyclic Systems with 2 Elements of Unsaturation
Stability Factors for Simple Dienes:
1. Isolated versus Conjugated: Conjugation stabilizes
2. Substitution: More highly substituted are more stable.
15.4 Stability of Allylic/Benzylic (Conjugated) Cations
Stability Factors for Cations:
1. Isolated versus Conjugated/Allylic: Conjugation stabilizes
2. Substitution: More highly substituted are more stable.
• Conjugation/allylic is more important than the substitution pattern of an isolated cation
(i.e. 1º allylic > 3º isolated)
Allylic Cations, Resonance, Charge Delocalization, and Allylic Symmetry/Asymmetry
a.
b.
c.
”Benzylic”
1. Two resonance structures each (at least)
2. Charge is delocalized, shared
3. Allylic cations can be symmetric or asymmetric
4. When an allylic cation is asymmetric, it’s helpful to evaluate which form would make a
larger contribution to the actual hybrid
• Cation substitution is more important than alkene substitution
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10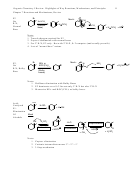 11
11 12
12 13
13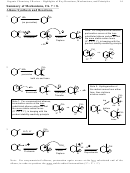 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26 27
27 28
28 29
29 30
30 31
31 32
32 33
33 34
34








