Review Of Organic Chemistry Page 9
ADVERTISEMENT
Organic Chemistry I Review: Highlights of Key Reactions, Mechanisms, and Principles
9
Ch. 7 Structure and Synthesis of Alkenes
C. E-Z Nomenclature (7-5)
• Each carbon of an alkene has two attachments.
1. Identify which of the two attachments on the left alkene carbon has higher priority.
2. Then identify which attachment on the right alkene carbon has higher priority.
“Z” (“zusammen” = “together”): the priority attachments are cis
•
• “E” (“entgegan = “opposite”): the priority attachments are trans
A
A
A
A
B
Z
B
B
B
2 common attachments
B
A
Z
Z ("together")
E (opposite)
no Stereo
When does E/Z apply?
1. If either alkene carbon has two common attachments, than stereo doesn’t apply
2. For tri- or tetrasubstituted alkenes (3 or 4 non-hydrogen attachments), E/Z must be used
if there is stereochemistry
3. For di-substituted alkenes (one H on each alkene carbon), either E/Z or cis/trans
designation can be used
7.7 Alkene Stability Pattern
C
C
C
C
C
H
C
H
C
H
C
C
H
H
>
C C
>
>
C C
C C
>
>
C C
C C
>
C C
C C
C
C
C
H
H
C
C
H
H
H
H
H
H
H
trans
1,1-
tetra-
tri-
mono-
cis
un-
disubbed
disubbed
subbed
subbed
subbed
disubbed
subbed
di-subbed
A. Increasing Substitution (# of non-hydrogens directly attached to alkene carbons) Increased
Stability
• Why? Electronic Reasons.
o Alkene carbons are somewhat electron poor due to the inferior overlap of pi bonds.
(One carbon doesn’t really “get” as much of the other carbon’s electron as is the
case in a nice sigma bond).
o Since alkyl groups are electron donors, they stabilize electron-deficient alkene
carbons.
o Analogous to why electron-donating alkyls give the 3º > 2º > 1º stability pattern for
cations and radicals
B. Trans is more stable than cis for 1,2-disubstituted alkenes
• Why?
o Steric Reasons
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10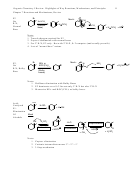 11
11 12
12 13
13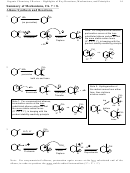 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26 27
27 28
28 29
29 30
30 31
31 32
32 33
33 34
34








