Review Of Organic Chemistry Page 3
ADVERTISEMENT
Organic Chemistry I Review: Highlights of Key Reactions, Mechanisms, and Principles
3
Stability/Reactivity/Selectivity Principles
1. Reactant Stability/Reactivity: The more stable the reactant, the less reactive it will be. In
terms of rates, this means that the more stable the reactant, the slower it will react. (The concept
here is that the more stable the reactant, the more content it is to stay as is, and the less
motivated it is to react and change into something different)
Key note: Often the “reactant” that’s relevant in this context will not be the original
reactant of the reaction, but will be the “reactant” involved in the rate determining step.
• Basicity
O
>
>
>
CH
Na
NHNa
ONa
2
ONa
A
C
B
D
Why: As anion stability increases from A to D, the reactivity decreases
• Nucleophilicity
O
>
>
>
CH
Na
NHNa
ONa
2
ONa
A
C
B
D
Why: As anion stability increases from A to D, the reactivity decreases
• Nucleophilicity
O
>
>
>
SeNa
SNa
ONa
ONa
A
C
B
D
Why: As anion stability increases from A to D, the reactivity decreases
• Reactivity toward alkanes via radical halogenation
F
> Cl
> Br
> I
because F• > Cl• > Br• > I•
2
2
2
2
Why: Chlorine is more reactive the bromine because chlorine radical is less stable then
bromine radical.
• Electrophilicity (Reactivity in S
2, S
1, E2, E1 Reactions)
N
N
>
I
>
Br
Cl
Why: As carbon-halogen bond stability increases, the reactivity decreases
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10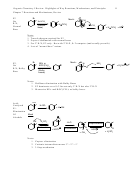 11
11 12
12 13
13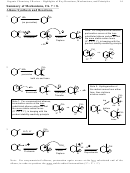 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26 27
27 28
28 29
29 30
30 31
31 32
32 33
33 34
34








