Review Of Organic Chemistry Page 32
ADVERTISEMENT
Organic Chemistry I Review: Highlights of Key Reactions, Mechanisms, and Principles
32
Seeing the Mechanism and Resonance Structures from Different Perspectives
H
E
1
E
H
E
E
H
H
1
1
1
H
H
E
H
H
-H
6
2
6
2
1
Addition to
H
H
H
H
5
3
Site 1
5
3
H
Resonance Pictures
H
4
4
H
H
1
1
E
E
E
H
H
H
E
-H
E
6
2
6
2
H
H
H
2
2
2
Addition to
H
H
H
H
5
3
5
3
Site 2
Resonance Pictures
H
H
4
4
H
H
1
1
H
H
E
H
H
6
2
6
2
-H
H
H
H
3
3
3
3
Addition to
H
H
E
E
E
H
E
5
3
Site 3
5
H
Resonance Pictures
H
4
4
H
H
1
1
H
H
H
H
-H
E
6
2
6
2
4
Addition to
H
E
H
H
E
E
H
H
4
H
H
4
4
5
3
5
3
Site 4
Resonance Pictures
H
E
4
H
H
1
1
H
H
E
H
H
6
2
6
2
-H
E
E
E
5
Addition to
H
H
H
H
H
5
E
H
5
5
3
Site 5
5
3
H
Resonance Pictures
H
4
4
H
H
1
H
1
H
H
6
6
6
H
H
E
H
-H
E
6
2
2
6
E
E
E
Addition to
H
H
H
H
5
3
5
3
Site 6
Resonance Pictures
H
H
4
4
NOTES:
1. These focus on drawing the resonance structures and seeing how the positive charge is
delocalized in the cation.
2. Notice that regardless of which position the electrophile adds to, the positive charge still ends up
delocalized onto the positions ortho and para relative to the site of addition
3. Notice that the site of addition does not have positive charge
4. Notice that the hydrogen that is lost is from the same carbon where the electrophile adds, not
from an ortho carbon
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10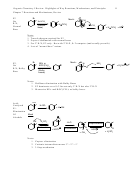 11
11 12
12 13
13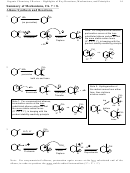 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26 27
27 28
28 29
29 30
30 31
31 32
32 33
33 34
34








