Review Of Organic Chemistry Page 28
ADVERTISEMENT
Organic Chemistry I Review: Highlights of Key Reactions, Mechanisms, and Principles
28
5 Major Aromatic Support Reactions
Ortho/Para
Activating/
Or Meta
Deactivating
Directing
Book
Fe, HCl
NO
NH
2
2
6
Activating
Ortho/Para
19.21
or
Sn, HCl
Reduction converts meta-director into an ortho-para director.
Fe, Sn, or several other reducing metals can work.
No mech required.
O
H H
Zn(Hg)
Activating
Ortho/Para
17.12
R
R
7
HCl
Clemmensen reduction converts meta-director into an ortho-para director.
Acylation (#4) followed by Clemmensen Reduction (#7) is the standard
method for introducing a 1º alkyl group. (Direct alkylation with a 1º alkyl
halide, reaction #3, fails due to cation rearrangement problems…)
No mech required.
17.4
SO
H
H
3
+
H
O, H
2
8
------
------
The sulfonyl group is a useful and reversible para-blocking group, since it can
be temporarily put on (reaction 5) but then can be removed later upon treatment
+
with H
O/H
(reaction 8).
2
The sulfonation/other reaction/desulfonation sequence is crucial for clean
ortho-substitution of an o/p director.
No mech required.
CH
1. KMnO
, NaOH
CO
H
3
4
2
9
Deactivating
Meta
17.14
+
2. H
O
3
Oxidation converts ortho/para-director into a meta-director.
Side alkyl chains longer than methyl can also be oxidized to benzoic acid in the
same way, although more time and heat is required.
For test purposes, just writing KMnO
will be OK. But the real reaction
4
requires a basic solution for the KMnO
to work, so an acidic workup step is
4
actually required to isolate the neutral carboxylic acid.
No mech required.
H
H
Br
Br
, hv or
H
2
------
------
17.14
peroxides
R
R
10
or NBS
Bromination occurs via free-radical mechanism.
It is selective for substitution at the benzylic position because the benzylic
radical intermediate is resonance-stabilized.
Note: keep distinct Br
/FeBr
from Br
/peroxides!
2
3
2
Product is subject to S
2 substitutions (benzylic bromides are especially good,
N
better than normal 2º bromides) and E2 eliminations with bulky bases.
“NBS” is N-bromosuccinimide, which functions just like Br
/peroxides, but is
2
much more convenient and cleaner because it avoids competing reactions
caused by lots of Br
and HBr.
2
Mech required.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10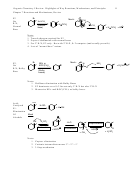 11
11 12
12 13
13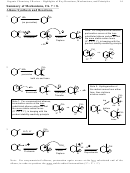 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26 27
27 28
28 29
29 30
30 31
31 32
32 33
33 34
34








