Review Of Organic Chemistry Page 15
ADVERTISEMENT
Organic Chemistry I Review: Highlights of Key Reactions, Mechanisms, and Principles
15
8
CH
3
Br
Br
2
(or Cl
)
H
2
Br
3 Notes
Br
Br
Br Br
1. Cation intermediate is cyclic
Br
bromonium (or chloronium) ion
H
Br
Cation
2. The nucleophile captures the
H
H
Capture
bromonium ion via backside attack
-this leads to the trans stereochemistry
3. The nucleophile attacks the bromonium
ion at the *more* substituted carbon
9
CH
3
Br
, H
O
OH
2
2
(or Cl
)
H
2
Br
H
OH
Br Br
O
2
OH
-H
H
Br
H
Br
Cation
Br
H
H
Capture
H
4 Notes
1. Cation intermediate is cyclic bromonium (or chloronium) ion
2. The nucleophile captures the bromonium ion via backside attack (ala SN2)
-this leads to the trans stereochemistry
3. The nucleophile attacks the bromonium ion at the *more* substituted carbon
-this explains the orientation (Markovnikov)
a. There is more + charge at the more substituted carbon
b. The Br-C bond to the more substituted carbon is a lot weaker
H
O
OH
More
-H
H
Substituted
Br
Br
End
H
CH
H
3
Br
Less
Substituted
H
End
Br
Br
H
-H
OH
O
H
H
H
4. Alcohols can function in the same way that water does, resulting in an ether OR rather than
alcohol OH.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10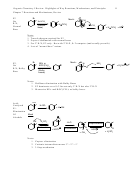 11
11 12
12 13
13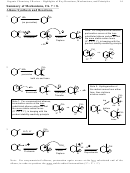 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26 27
27 28
28 29
29 30
30 31
31 32
32 33
33 34
34








