Review Of Organic Chemistry Page 8
ADVERTISEMENT
Organic Chemistry I Review: Highlights of Key Reactions, Mechanisms, and Principles
8
6.16 Comparing S
2 vs S
1
N
N
S
1
S
2
N
N
1
Nucleophile
Neutral, weak
Anionic, strong
2
Substrate
3º R-X > 2º R-X
1º R-X > 2º R-X
Allylic effect…
Allylic Helps
Allylic helps
3
Leaving Group
I > Br > Cl
I > Br > Cl
4
Solvent
Polar needed
Non-factor
5
Rate Law
K[RX]
k[RX][Anion]
6
Stereochemistry
Racemization
Inversion
(on chiral, normally 2º R-X)
7
Ions
Cationic
Anionic
8
Rearrangements
Problem at times
Never
6.21 Comparing E2 vs E1
E1
E2
1
Nucleophile/Base
Neutral, weak, acidic
Anionic, strong, basic
2
Substrate
3º R-X > 2º R-X
3º RX > 2º RX > 1º RX
Allylic effect…
Allylic Helps
Non-factor
3
Leaving Group
I > Br > Cl
I > Br > Cl
4
Solvent
Polar needed
Non-factor
5
Rate Law
K[RX]
k[RX][Anion]
6
Stereochemistry
Non-selective
Trans requirement
7
Ions
Cationic
Anionic
8
Rearrangements
Problem at times
Never
9
Orientation
Zaitsev’s Rule: Prefer
Zaitsev’s Rule: Prefer more
more substituted alkene
Substituted alkene (assuming
trans requirement permits)
Comparing S
2 vs S
1 vs E2 vs E1: How Do I Predict Which Happens When?
N
N
Step 1: Check nucleophile/base.
• If neutral, then S
1/E1 mixture of both
N
• If anionic, then S
2/E2.
N
Step 2: If anionic, and in the S
2/E2 pool, then Check the substrate.
N
o 1º S
2
N
o 2º S
2/E2 mixture. Often more S
2, but not reliable…
N
N
o 3º E2
Notes:
1º R-X
S
2 only
No E2 or S
1/E1 (cation too
N
N
lousy for S
1/E1; S
2 too fast
N
N
for E2 to compete)
3º R-X
E2 (anionic) or
No S
2 (sterics too lousy)
N
S
1/E1 (neutral/acidic)
N
2º R-X
mixtures common
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10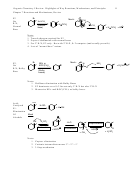 11
11 12
12 13
13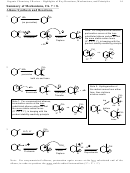 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26 27
27 28
28 29
29 30
30 31
31 32
32 33
33 34
34








