Review Of Organic Chemistry Page 19
ADVERTISEMENT
Organic Chemistry I Review: Highlights of Key Reactions, Mechanisms, and Principles
19
Impact of Allylic Cation Resonance on Reactivity and Product Formation
1. Rates: Resonance/conjugation stability enhances rates when cation formation is rate-
determining
2. Product Distribution: Product mixtures often result if an allylic cation is asymmetric.
• The two different resonance structures can lead to different products.
• When two isomeric products can form from an allylic cation, consider two things:
1. Which product is more stable?
• This will impact “product stability control” = “thermodynamic control” =
“equilibrium control”
• To assess product stability, focus on the alkene substitution
2. Which resonance form of the cation would have made a larger contribution?
• This will often favor “kinetic control”, in which a product which may not ultimately
be the most stable forms preferentially
3. Position of Cation Formation: When a conjugated diene is protonated, consider which site of
protonation would give the best allylic cation.
Sections 15.5,6 1,2 vs. 1,4 Addition to Conjugated Dienes: “Kinetic” vs. “Thermodynamic”
Control
Note: “Thermodynamic Control” = “Product-Stability Control” = “Equilibrium Control”
This is when the most stable of two possible products predominates. Either of two factors can
cause this:
o Transition State:
The most stable product is formed fastest via the most stable
transition state (normally true, but not always)
o Equilibrium: Even if the most stable product is not formed fastest, if the two products
can equilibrate, then equilibrium will favor the most stable product
Kinetic Control: If the less stable of two possible products predominates.
This will always require that for some reason the less stable product forms via a better transition
state (transition-state stability/reactivity principle). Common factors:
o Charge distribution in an allylic cation or radical. The position of charge in the major
resonance contributor may lead to one product, even though it may not give the most
stable product.
o Proximity of reactants. In an H-X addition to a diene, often the halide anion is closer to
one end of the allylic cation than the other, resulting in “1,2 addition” over “1,4
addition”.
o Steric factors. With a bulky E2 base, for example, the transition state leading to what
would be the more stable Zaytsev alkene has steric problems, so it gives the Hoffman
alkene instead.
15.7 Allylic/Benzylic Radicals
Stability Factors for Radicals:
1. Isolated versus Conjugated/Allylic: Conjugation stabilizes
2. Substitution: More highly substituted are more stable.
• Conjugation/allylic is more important than the substitution pattern of an isolated cation
Impact of Radical Resonance on Reactivity and Product Formation
1. Rates:
2. Product Distribution: Product mixtures often result if an allylic radical is asymmetric.
3. Position of Radical Formation
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10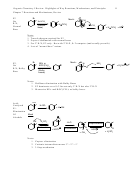 11
11 12
12 13
13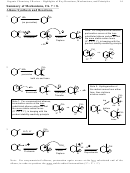 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26 27
27 28
28 29
29 30
30 31
31 32
32 33
33 34
34








