Review Of Organic Chemistry Page 2
ADVERTISEMENT
Organic Chemistry I Review: Highlights of Key Reactions, Mechanisms, and Principles
2
4.16 Reactive Intermediates: Stability Patterns
• Shortlived, unstable, highly reactive intermediates
• Normally lack normal bonding
These are tremendously important:
1. They will be the least stable intermediate in any multistep mechanism
2. When formed, they are products of the rate-determining step
3. Factors that stabilize them will speed up reaction rates
Thus it is very important to know their stability patterns!
Class
Structure
Stability Pattern
Carbocations
Allylic > 3º > 2º > 1º > methyl > alkenyl
Electron
Electrophilic/
C
(vinyl, aryl)
Poor
Acidic
Carbon
Allylic > 3º > 2º > 1º > methyl > alkenyl
Electron
Electrophilic/
C
Radicals
(vinyl, aryl)
Poor
Acidic
Carbanions
Allylic > alkenyl (vinyl, aryl) > methyl > 1º >
Electron
Nucleophilic/
C
2º > 3º
Rich
Basic
Notes
1. Both carbocations and radicals have the same pattern. So you don’t need to memorize them
twice!
2. Carbanions are almost exactly the reverse, except that being allylic is ideal for both.
3. All benefit from resonance (allylic).
4. Cations and radicals both fall short of octet rule. As a result, they are both electron deficient.
Carbanions, by contrast, are electron rich.
5. Alkyl substituents are electron donors. As a result, they are good for electron deficient cations
and radicals (3º > 2º > 1º > methyl) but bad for carbanions.
6. Alkenyl (vinyl or aryl) carbons are inherently a bit electron poor.
This is excellent for
carbanions, but terrible for cations or radicals.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10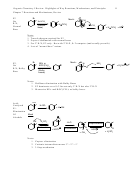 11
11 12
12 13
13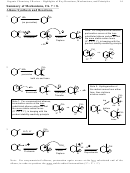 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26 27
27 28
28 29
29 30
30 31
31 32
32 33
33 34
34








