Review Of Organic Chemistry Page 30
ADVERTISEMENT
Organic Chemistry I Review: Highlights of Key Reactions, Mechanisms, and Principles
30
Formation of the Active Electrophiles
1. In each case, the cationic form of the thing that adds must be generated
2. The arrow pushing in the E+ generation always involves an arrow going from the cation
precursor to the Lewis or Bronsted acid
3. For class, we will focus on sulfuric acid as Bronsted acid, and AlCl
or FeBr
as Lewis acids
3
3
But in an actual synthesis lab, other Bronsted or Lewis acids are available and may
sometimes provide superior performance.
Cation
Needed
Br
H
FeBr
3
Br Br
Br
FeBr
(cat.)
Br
FeBr
3
Br
3
+ Br
(+ HBr)
1
2
(or Fe cat)
H
Cl
AlCl
3
Cl Cl
Cl
Cl
AlCl
AlCl
(cat.)
3
Cl
+ Cl
3
(+ HCl)
2
NO
H
2
H
SO
H
SO
2
4
2
4
NO
(+ H
O)
2
+ HNO
NO
2
O
N OH
+ H
O + HSO
2
2
3
2
4
2
H
R
AlCl
(cat.)
AlCl
3
3
3
+
R-X
R X
R
X
AlCl
3
R
O
O
O
AlCl
3
H
O
O
AlCl
(cat.)
3
Cl
AlCl
R
R
Cl
3
R
4
+
Cl
R
R
H
SO
H
H
SO
H
SO
3
2
4
2
4
5
+
+ HSO
SO
SO
SO
H
3
4
3
3
SO
H
3
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10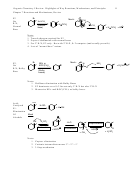 11
11 12
12 13
13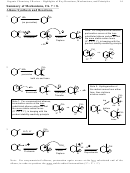 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26 27
27 28
28 29
29 30
30 31
31 32
32 33
33 34
34








