Review Of Organic Chemistry Page 20
ADVERTISEMENT
Organic Chemistry I Review: Highlights of Key Reactions, Mechanisms, and Principles
20
Section 15.10 S
2 on Allylic, Benzylic Systems Are Really Fast
N
Ex.
NaOCH
3
Slow, and contaminated by competing E2
10 hours
Br H
H OCH
3
80% yield
NaOCH
Fast and Clean
3
15 min
Br H
H OCH
3
100% yield
Why? Because the backside-attack transition-state is stabilized by conjugation!
(Transition state-stability-reactivity principle).
Br
Br H
H
H OCH
H
CO
3
3
1. Neither the product nor the reactant has conjugation, so it’s hard to see why conjugation should
apply
2
2. However, in the 5-coordinate T-state the reactive carbon is sp
hybridized
the nucleophile and the electrophile are essentially on opposite ends of a temporary p-orbital.
2
3. That transient sp
hybridization in the transition-state is stabilized by π-overlap with the adjacent
p-bond.
4. This stabilization of the transition-state lowers the activation barrier and greatly accelerates
reaction
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10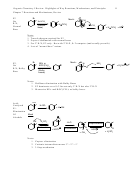 11
11 12
12 13
13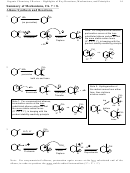 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26 27
27 28
28 29
29 30
30 31
31 32
32 33
33 34
34








