Chem 161 Chapter 9 Molecular Geometry Worksheet - Shape Determines Function Page 11
ADVERTISEMENT
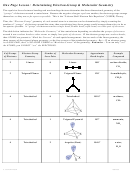
Guidelines for Applying the VSEPR Model
1. Draw Lewis formula
2. Count number of outer atoms and lone pairs around central atom (treating double and
triple bonds as single bonds) to get the General Formula
– Match the General Formula (AB
E
) to get the Molecular Geometry.
x
y
– Remember that lone pair electrons occupy more space than bonded pairs of electrons,
so the bond angles are compressed when the central atom has lone pairs.
Ex:
For each of the following,
i. Draw the Lewis structure, including resonance structures if they apply, showing all
non-zero formal charges and minimizing formal charges.
ii. Indicate the molecular geometry (shape) and the bond angles. Sketch the molecule.
–
PF
:
I
:
3
3
shape = _________________________
shape = ______________________________
bond angle(s) = ___________________
bond angle(s) = ___________________
–
–
NH
:
SCN
:
2
shape = _________________________
shape = ______________________________
bond angles = ____________________
bond angles = _________________________
–
SeF
:
IF
:
4
4
shape = _________________________
shape = ______________________________
bond angles = ____________________
bond angles = _________________________
CHEM
1 61:
C hapter
9
page
1 1
o f
4 0
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26 27
27 28
28 29
29 30
30 31
31 32
32 33
33 34
34 35
35 36
36 37
37 38
38 39
39 40
40