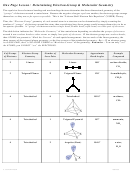
Chem 161 Chapter 9 Molecular Geometry Worksheet - Shape Determines Function Page 16
ADVERTISEMENT
9.4 VALENCE BOND THEORY
Localized Electron Model
– a molecule is a collection of atoms that share electrons between their atomic orbitals
– “localized” because the electrons are assumed to exist between the atoms
Valence Bond (VB) Theory
– assumes electrons in molecules occupy atomic orbitals of individual atoms in a bond
– accounts for changes in PE as distance between reacting atoms changes
– incomplete picture of all aspects of bonding but gives some insight
Basic Theory
– a covalent bond consists of a pair of electrons in atomic orbitals
1
Ex. 1 H atom has the electron configuration:
1s
H atom has the atomic orbital diagram:
↑
1s
Lewis formula for H
molecule: H—H
2
Each H in a H
molecule has the atomic orbital diagram:
↑↓
2
1s
S orbitals are spherical, so two 1s orbitals for the two H atoms in H
bond by
2
having an overlapping region where the two electrons are shared.
2
2
5
Ex. 1
F atom has the electron configuration:
1s
2s
2p
F atom has the atomic orbital diagram:
.
.
.
↑↓
↑↓
↑
2p
.
↑↓
F atoms forming F
molecule:
2s
2
↑↓
••
••
••
••
•
•
1s
• •
•
•
•
F
+
F
F F
→
•
•
•
•
•
••
••
••
••
CHEM
1 61:
C hapter
9
page
1 6
o f
4 0
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26 27
27 28
28 29
29 30
30 31
31 32
32 33
33 34
34 35
35 36
36 37
37 38
38 39
39 40
40