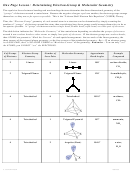
Chem 161 Chapter 9 Molecular Geometry Worksheet - Shape Determines Function Page 34
ADVERTISEMENT
Electron Configurations for Molecules using MO Diagrams
Just like for individual atoms, electron configurations can be written for molecules, and the
electron configurations are based on the occupied orbitals in the molecule’s MO diagram.
Since there are 2 electrons in the
molecular orbital for the H
molecules, its electron
σ
1s
2
configuration shows the
MO in parentheses followed by the superscript 2.
σ
1s
2
Electron configuration for H
:
(
)
σ
1s
2
Bond Order and Bond Strength
– MO Theory allows determination of bond order, a measure of the relative bond strength.
– The higher the bond order, the stronger the bond.
– The bond order is given as follows (with the number of electrons being divided by 2 since
bonds consist of electron pairs).
#
of
bonding
electrons
#
of
antibondin
g
electrons
−
bond order =
2
Ex. 1: Use the MO diagram for H
to calculate its bond order.
2
Ex. 2: Consider the He atom and why the He
molecule does not form.
2
a. Write the electron configuration for helium: ______________________
b. Fill in the MO diagram for the He
molecule below:
2
CHEM
1 61:
C hapter
9
page
3 4
o f
4 0
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9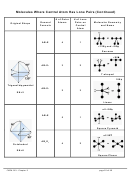 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26 27
27 28
28 29
29 30
30 31
31 32
32 33
33 34
34 35
35 36
36 37
37 38
38 39
39 40
40