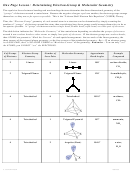
Chem 161 Chapter 9 Molecular Geometry Worksheet - Shape Determines Function Page 33
ADVERTISEMENT
9.7 MOLECULAR ORBITAL (MO) THEORY
Problems with the Localized Electron Model
– Does not address resonance
– Does not correctly explain molecules with unpaired electrons (paramagnetic molecules)
– Provides not information on bond energies (i.e., why some molecules don’t form)
Molecular Orbital (MO) Model (or MO Theory)
– Molecules exist as positively charged nuclei sharing all of the electrons within the molecule
– Electrons are contained within “molecular orbitals”, not localized between 2 atoms.
– MO Theory correctly predicts relative bond strength, magnetism, and bond polarity.
Characteristics of Molecular Orbitals (MOs) are similar to atomic orbitals.
– Each molecular orbital can hold 2 electrons.
– Pauli Exclusion Principle applies → electrons in same MO must have opposite spins.
– Hund’s Rule applies → one e
s in each degenerate orbital with same spin before pairing.
−
2
– The square of the MO wave function (ψ
) gives the probability density or “electron cloud”
– Like hybrid atomic orbitals, the # of MOs formed equals the original # of atomic orbitals.
– When 2 atoms form a molecule, their electrons can only occupy the new MOs.
*
1s atomic orbitals
2 new MOs: bonding
and antibonding
σ
σ
→
s 1
s 1
We can also show the formation of the MOs using orbitals diagrams and H
as an example.
2
Isolated H atoms
are shown in blue, and the
H
molecule
is shown in red.
2
The two 1s orbitals from each H atom form two new molecular orbitals:
and
σ
σ
*
1s
s 1
– Because the lower MO results from direct overlap, it is called a bonding
MO.
σ
s 1
– A bonding MO is lower in energy than the original atomic orbitals.
– A node between the nuclei in the upper MO results in a antibonding
MO.
σ
*
s 1
– An antibonding MO is higher in energy than the original atomic orbitals.
– Aufbau (Building-Up) Principle applies → bonding
σ
MO fills before antibonding
σ
*
MO.
1s
s 1
CHEM
1 61:
C hapter
9
page
3 3
o f
4 0
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26 27
27 28
28 29
29 30
30 31
31 32
32 33
33 34
34 35
35 36
36 37
37 38
38 39
39 40
40