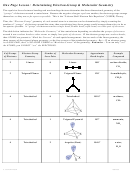
Chem 161 Chapter 9 Molecular Geometry Worksheet - Shape Determines Function Page 32
ADVERTISEMENT
atomic orbital diagram each N in N
atomic orbital diagram for each O in O
2
2
Magnetic Properties of Molecules
paramagnetic: substance contains unpaired electrons
weakly attracted to magnetic field
→
diamagnetic: substance contains only paired electrons
slightly repelled by a magnetic field
→
Ex. 5: Based on Ex. 4, the N
molecule is __________,
paramagnetic
diamagnetic
2
and the O
molecule is __________.
paramagnetic
diamagnetic
2
Ex. 6: Based on your answers to Ex. 5 above, predict what will happen when liquid nitrogen is
poured over magnetic field. Will it stick or not? Explain.
Ex. 7: Based on your answers to Ex. 5 above, predict what will happen when liquid oxygen is
poured over magnetic field. Will it stick or not? Explain.
Ex. 8: Based on the YouTube video showing the magnetic properties of liquid N
and liquid O
,
2
2
the N
molecule is __________,
paramagnetic
diamagnetic
2
and the O
molecule is __________.
paramagnetic
diamagnetic
2
CHEM
1 61:
C hapter
9
page
3 2
o f
4 0
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9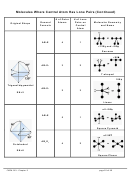 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26 27
27 28
28 29
29 30
30 31
31 32
32 33
33 34
34 35
35 36
36 37
37 38
38 39
39 40
40