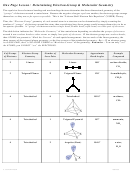
Chem 161 Chapter 9 Molecular Geometry Worksheet - Shape Determines Function Page 7
ADVERTISEMENT
Molecules Where Central Atom Has One Or More Lone Pairs
From Trigonal Planar (SN=3)
AB
E: bent
2
– start with AB
molecule (trigonal planar) and replace a B atom with a lone pair
3
– bond angles are now <120°
From Tetrahedral (SN=4)
AB
E: trigonal pyramidal (central atom + 3 outer atoms make a pyramid)
3
– start with AB
molecule (tetrahedral) and replace a B atom with lone pair
4
– bond angles are now less than 109.5°
AB
E
:
bent
2
2
– start with AB
molecule (tetrahedral) and replace 2 B atoms with 2 lone pairs
4
From Trigonal Bipyramidal (SN=5)
AB
E: seesaw
4
– start with AB
molecule and replace one B atom with one lone pair
5
– an outer atom can be taken from an axial or an equatorial position
– from axial: lone pair is 90° from equatorial atoms and 180° from other axial atom
– from equatorial: lone pair is 90° from two axial and 120° from two other
equatorial B atoms
– taking the outer atom from the equatorial position maximizes the distance between
the lone pair (lp) and bonding pairs (bp)
– bond angles are now <90° and <120°
CHEM
1 61:
C hapter
9
page
7
o f
4 0
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9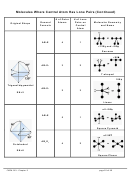 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26 27
27 28
28 29
29 30
30 31
31 32
32 33
33 34
34 35
35 36
36 37
37 38
38 39
39 40
40