Chem 161 Chapter 9 Molecular Geometry Worksheet - Shape Determines Function Page 4
ADVERTISEMENT
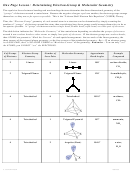
Molecular Geometries with 2 to 6 Outer Atoms on the Central Atom
(where the Central Atom Has No Lone Pairs)
Consider a molecule composed of only two types of atoms, A and B:
A=central atom
B=outer atoms
For three or more atoms in a molecule, general formula: AB
(where #=2 to 6)
#
AB
: linear
2
– the two outer atoms are 180° from each other
– steric number (SN) = 2
AB
: trigonal planar
3
– three outer atoms at the corners of an equilateral triangle
– each outer atom is 120° from the other two outer atoms
– SN = 3
AB
: tetrahedral (tetra = four) since four-sided, or four faces
4
– maximum distance between electrons requires 3D
structure with 109.5° bond angles
– each outer atom is 109.5° from the other outer atoms
AB
: trigonal bipyramidal
5
– trigonal = three outer atoms form a planar triangle around
central atom
– bipyramidal = two outer atom directly above and below central
atom, connecting outer atom forms two 3-sided pyramids
– 3 outer atoms are at equatorial positions, 120° from one another at the ends of a
planar triangle
– 2 outer atoms are at axial positions, above and below central
atom and 90° from the equatorial atoms
AB
: octahedral (octa=eight) connecting the B atoms → eight faces
6
– all outer atoms are 90° away from each other
– terms "axial" and "equatorial" do not apply because all six
positions are identical
CHEM
1 61:
C hapter
9
page
4
o f
4 0
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9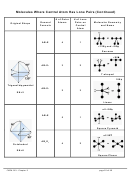 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26 27
27 28
28 29
29 30
30 31
31 32
32 33
33 34
34 35
35 36
36 37
37 38
38 39
39 40
40