Chem 161 Chapter 9 Molecular Geometry Worksheet - Shape Determines Function Page 3
ADVERTISEMENT
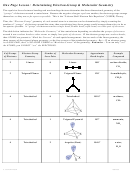
Molecule #4: PCl
5
–
a. Draw the Lewis structure below.
b. To maximize the distance between e
pairs,
electrons are 90˚ or 120˚ away from each other.
Lewis structure
P
Thus, this shape is called trigonal bipyramidal,
where the bond angles are 120˚ for equatorial
atoms and 90˚ for axial atoms.
3. Note: Three of the outer atoms make a triangle and sit in the same plane with the central
atom while one atom sits above the plane and another sits below.
– Connecting the outer atoms makes two pyramids
the name trigonal bipyramidal.
→
In this shape, the four atoms in the center (in the equator or equatorial positions) have
120˚ bond angles while the atoms above (on each axis or axial positions) and below form
90˚ bond angles with the equatorial atoms.
Molecule #5:
SF
6
–
1. Draw the Lewis formula below.
b. To maximize the distance between e
pairs,
electrons are 90˚ away from each other.
Lewis structure
S
Thus, this shape is called octahedral, where
the bond angles are all 90˚.
3. Note that four of the outer atoms make a square and all sit in the same plane with the
central atom while one atom sits above the plane and another sits below. Connecting all the
outer atoms makes 8 faces or sides, so this shape is called octahedral.
Note that all the atoms make right angles to one another, so all the bond angles are 90˚.
CHEM
1 61:
C hapter
9
page
3
o f
4 0
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26 27
27 28
28 29
29 30
30 31
31 32
32 33
33 34
34 35
35 36
36 37
37 38
38 39
39 40
40