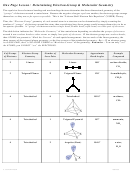
Chem 161 Chapter 9 Molecular Geometry Worksheet - Shape Determines Function Page 36
ADVERTISEMENT
*
2p
and 2p
atomic orbitals
4 new MOs: two bonding
and two antibonding
,
π
π
→
2p
z
y
2p
which are each offset from the 2p
MOs and one another by 90
.
x
°
– Because the lower MO results from indirect overlap, it is called a bonding
π
MO.
2p
– A bonding MO is lower in energy than the original atomic orbitals.
*
– A node between the nuclei in the upper MO results in a antibonding
MO.
π
2p
– An antibonding MO is higher in energy than the original atomic orbitals.
The following molecular orbital diagram shows the energy levels resulting from two sets of 2s
and 2p atomic orbitals:
– Because of the direct (or head-on) overlap in the bonding
σ
, the interaction between
2p
the 2p
molecular orbitals is stronger than in the 2p
and 2p
, so the bonding
is lower in
σ
x
x
y
2p
energy than either of the bonding
molecular orbitals.
π
2p
CHEM
1 61:
C hapter
9
page
3 6
o f
4 0
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9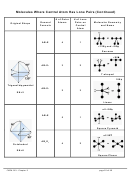 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26 27
27 28
28 29
29 30
30 31
31 32
32 33
33 34
34 35
35 36
36 37
37 38
38 39
39 40
40