Chem 161 Chapter 9 Molecular Geometry Worksheet - Shape Determines Function Page 12
ADVERTISEMENT
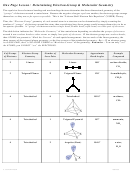
9.5 SHAPES AND BONDING IN LARGER MOLECULES
Geometry of Molecules with More than One Central Atom
– Overall geometry of entire molecule cannot be described
instead describe the shape around each central atom in molecule
→
– e.g. CH
OH
3
Lewis structure
H
H
C
H
H—C—O—H
O
H
H
H
tetrahedral around C and
bent around O
bond angle = 109.5°
bond angle = <109.5°
Ex. What is the geometry around each central atom in acetic acid, CH
COOH?
3
H
O
st
1
C: shape = __________________, bond angles = _______
nd
2
C: shape = __________________, bond angles = _______
H—C—C—O—H
O: shape = _____________________, bond angle = _______
H
Sketch the 3D shape
(with approximate bond
angles) for acetic acid
at the right:
9.3 POLAR BONDS AND POLAR MOLECULES
Bonding electrons are not always shared equally
– If two atoms bonded together have different electronegativity (EN) values
→ separation of charges = dipole
..
. .
→ more EN atom gains a partial negative charge
H―F
→ other atom gains a partial positive charge
..
– molecules that have a dipole are polar molecules
– A dipole is indicated with an arrow pointing to more EN atom.
– The more polar the molecule, the greater its dipole moment, the quantitative
measurement of the separation of positive and negative charges in the molecule.
For diatomic molecules:
– nonpolar molecules: when the 2 atoms have equal EN values → no dipole
– polar molecules: when the 2 atoms have different EN values → dipole
CHEM
1 61:
C hapter
9
page
1 2
o f
4 0
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26 27
27 28
28 29
29 30
30 31
31 32
32 33
33 34
34 35
35 36
36 37
37 38
38 39
39 40
40