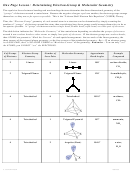
Chem 161 Chapter 9 Molecular Geometry Worksheet - Shape Determines Function Page 19
ADVERTISEMENT
Case #2: BH
3
1. Draw the Lewis formula for the BH
molecule below:
3
Lewis formula
Sketch of molecule
2. BH
’s shape is ______________________ and its bond angles are _______.
3
Sketch the molecule above.
3. Electron configuration for B (using core notation): ___________________
4. Draw the atomic orbital diagram for the valence electrons in B:
5. Promote electrons in the atomic orbital diagram for B, so B can form 3 bonds.
Although B now has unpaired electrons in 3 orbitals for bonding, the atomic orbital diagram
indicates that two of the B–H bonds (from 2p) should be the same but one (from 2s) should
be different.
But VSEPR predicts BH
is trigonal planar and experimental evidence indicates that the
3
three B–H bonds in BH
are equivalent
3
2
→ hybridize one 2s and two 2p orbitals to get 3 equivalent sp
hybrid orbitals
Show the atomic orbital diagrams for the hybridization below:
hybridization
"
" " " " "
→
€
atomic orbital diagram before hybridizing
atomic orbital diagram after hybridizing
CHEM
1 61:
C hapter
9
page
1 9
o f
4 0
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26 27
27 28
28 29
29 30
30 31
31 32
32 33
33 34
34 35
35 36
36 37
37 38
38 39
39 40
40