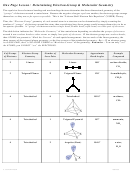
Chem 161 Chapter 9 Molecular Geometry Worksheet - Shape Determines Function Page 30
ADVERTISEMENT
6. Show the atomic orbital diagrams for the formation of the three hybrid orbitals for each C
atom below:
hybridization
"
" " " " "
→
€
atomic orbital diagram before hybridizing
atomic orbital diagram after hybridizing
Each C–H bond and the first C–C bond are all sigma bonds, as shown below:
Note: The atomic orbital diagram for each C atom drawn in step 6 C shows two unhybridized p
nd
rd
orbitals. The 2
and 3
C–C bonds form from the electrons in the unhybridized p orbitals.
– Each carbon has two unpaired electrons in unhybridized p orbitals (in the 2p
and 2p
),
y
z
which are perpendicular to the sp orbitals forming the C–H and first C–C bond.
– These two electrons in the 2p
and 2p
bond to each other via a sideways overlap to make
y
z
nd
rd
the 2
and 3
bond in the triple bond, as shown above.
Note: The carbon-carbon triple bond in C
H
consists of a σ bond (shown in blue) and two
2
2
carbon-carbon π bonds (shown in green) made from the four lobes on the unhybridized p’s.
Example: HCN
1. Draw the Lewis structure for HCN:
2. The shape around C is ______________, and the bond angle is __________.
3. Electron configuration for C: ___________________
4. Given the shape around each C atom, C has _________ atomic hybrid orbitals.
5. Draw the atomic orbital diagram for
the valence electrons in C, promoting
electrons, so C can form 4 bonds.
CHEM
1 61:
C hapter
9
page
3 0
o f
4 0
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9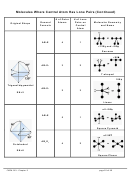 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26 27
27 28
28 29
29 30
30 31
31 32
32 33
33 34
34 35
35 36
36 37
37 38
38 39
39 40
40