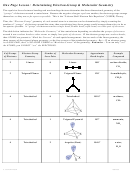
Chem 161 Chapter 9 Molecular Geometry Worksheet - Shape Determines Function Page 13
ADVERTISEMENT
For molecules of three of more atoms:
– polarity depends on the number of outer atoms and lone pairs around the central atom
Example: Determine whether the following have dipole moments:
BeCl
:
H
O:
O
2
2
—
—
Cl
Be
Cl
H
H
CCl
versus CHCl
:
4
3
H
CCl 4
CHCl 3
Ex. 2: For each of the following molecules,
i. Draw the Lewis structure (including resonance structures when necessary).
ii. Determine its molecular geometry and bond angle(s).
iii. Sketch the molecule’s 3D shape, draw a dipole arrow for each polar covalent bond.
iv. Indicate if the molecules is polar or nonpolar.
a. SF
4
Lewis formula
sketch of 3D shape with dipoles
Molecular geometry: ______________________
SF
is __________.
4
Bond angles: ___________________________
(Circle one) polar
nonpolar
CHEM
1 61:
C hapter
9
page
1 3
o f
4 0
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9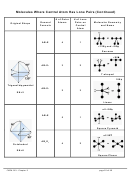 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26 27
27 28
28 29
29 30
30 31
31 32
32 33
33 34
34 35
35 36
36 37
37 38
38 39
39 40
40